23andMe Investor Presentation Deck
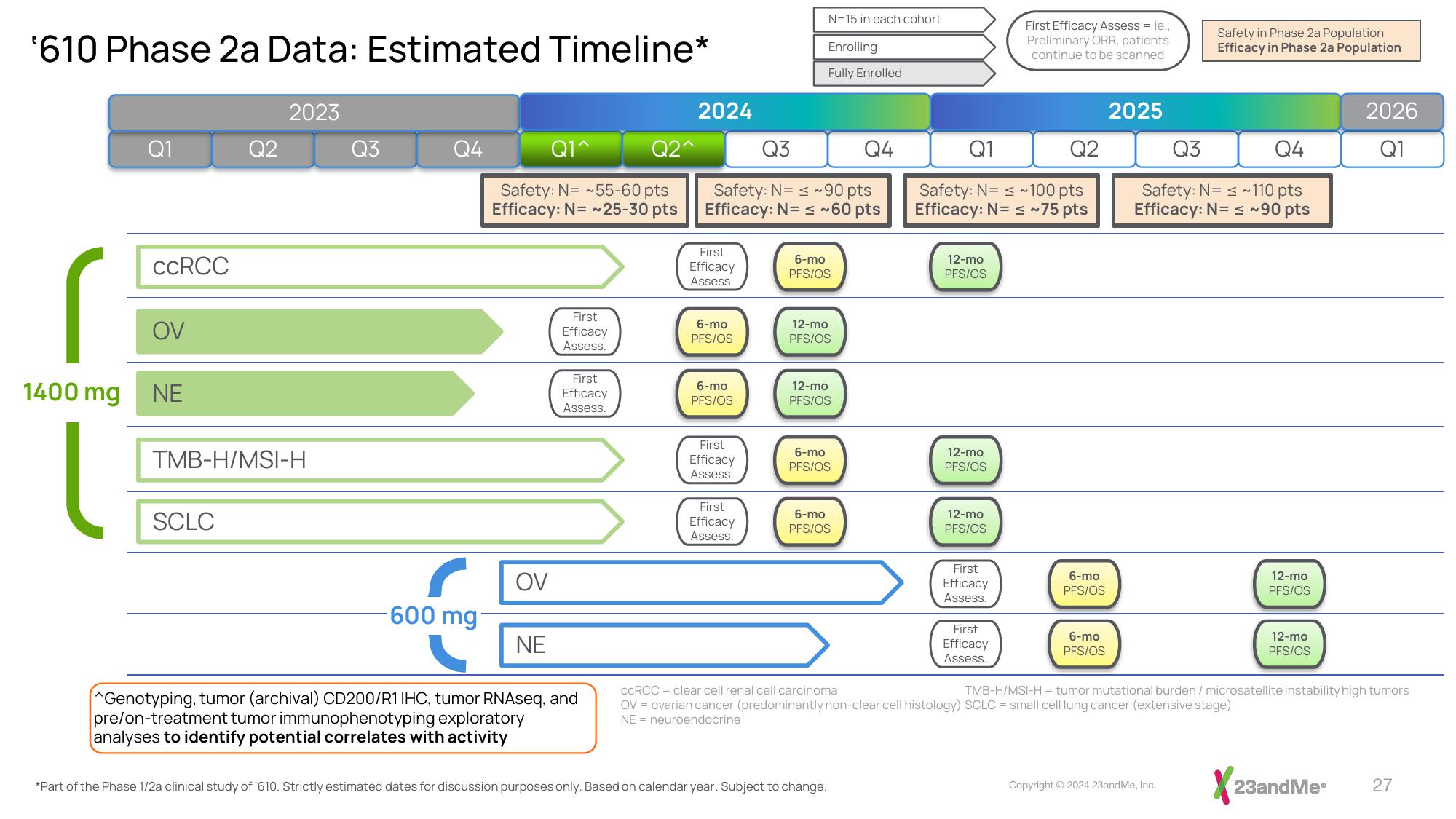
'610 Phase 2a Data: Estimated Timeline*
1400 mg
Q1
CCRCC
OV
NE
Q2
SCLC
2023
TMB-H/MSI-H
Q3
Q4
-600 mg
Q1^
Q2
Safety: N= ~55-60 pts
Efficacy: N= ~25-30 pts
OV
NE
First
Efficacy
Assess.
First
Efficacy
Assess.
^Genotyping, tumor (archival) CD200/R1 IHC, tumor RNAseq, and
pre/on-treatment tumor immunophenotyping exploratory
analyses to identify potential correlates with activity
2024
First
Efficacy
Assess.
Q3
Q4
Safety: N= ~90 pts
Efficacy: N= ≤ ~60 pts
6-mo
PFS/OS
6-mo
PFS/OS
First
Efficacy
Assess.
First
Efficacy
Assess.
N=15 in each cohort
Enrolling
Fully Enrolled
6-mo
PFS/OS
12-mo
PFS/OS
12-mo
PFS/OS
6-mo
PFS/OS
6-mo
PFS/OS
*Part of the Phase 1/2a clinical study of '610. Strictly estimated dates for discussion purposes only. Based on calendar year. Subject to change.
Q1
Q2
Safety: N= ≤ ~100 pts
Efficacy: N= ≤ ~75 pts
12-mo
PFS/OS
12-mo
PFS/OS
12-mo
PFS/OS
First
Efficacy
Assess.
First Efficacy Assess = ie.,
Preliminary ORR, patients
continue to be scanned
First
Efficacy
Assess.
6-mo
PFS/OS
6-mo
PFS/OS
2025
Safety in Phase 2a Population
Efficacy in Phase 2a Population
Q3
Q4
Safety: N= ≤ ~110 pts
Efficacy: N= ≤ ~90 pts
CCRCC = clear cell renal cell carcinoma
OV = ovarian cancer (predominantly non-clear cell histology) SCLC = small cell lung cancer (extensive stage)
NE neuroendocrine
Copyright © 2024 23andMe, Inc.
12-mo
PFS/OS
12-m
PFS/OS
TMB-H/MSI-H = tumor mutational burden / microsatellite instability high tumors
2026
Q1
23and Me
27View entire presentation