BenevolentAI Investor Day Presentation Deck
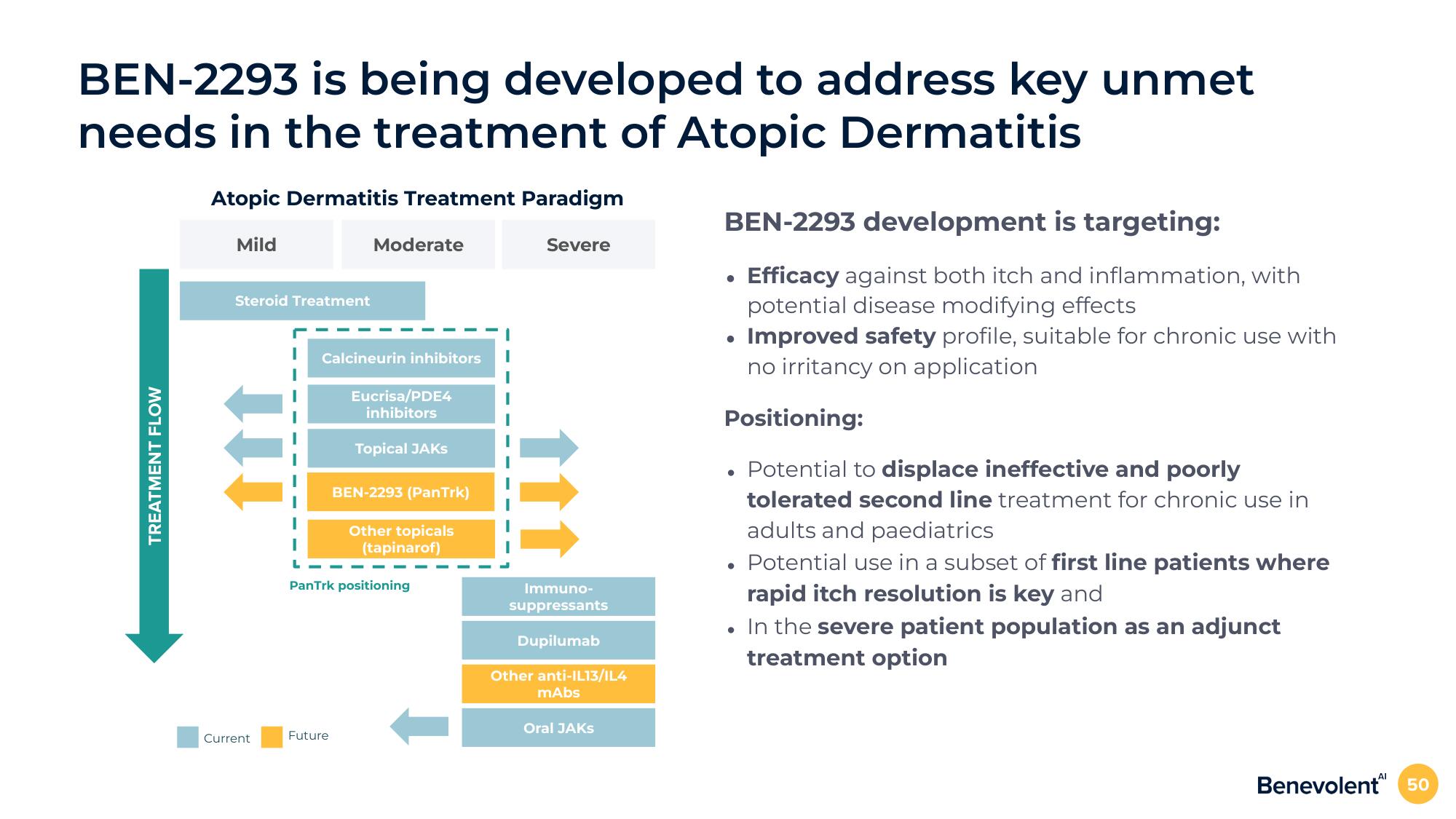
BEN-2293 is being developed to address key unmet
needs in the treatment of Atopic Dermatitis
TREATMENT FLOW
Atopic Dermatitis Treatment Paradigm
Mild
Steroid Treatment
Current
Moderate
Calcineurin inhibitors
Future
Eucrisa/PDE4
inhibitors
Topical JAKS
BEN-2293 (Pan Trk)
Other topicals
(tapinarof)
L1_
PanTrk positioning
Severe
Immuno-
suppressants
Dupilumab
Other anti-IL13/IL4
mAbs
Oral JAKS
BEN-2293 development is targeting:
Efficacy against both itch and inflammation, with
potential disease modifying effects
Improved safety profile, suitable for chronic use with
no irritancy on application
●
●
Positioning:
Potential to displace ineffective and poorly
tolerated second line treatment for chronic use in
●
●
adults and paediatrics
Potential use in a subset of first line patients where
rapid itch resolution is key and
In the severe patient population as an adjunct
treatment option
Benevolent 50View entire presentation