BioNTech Results Presentation Deck
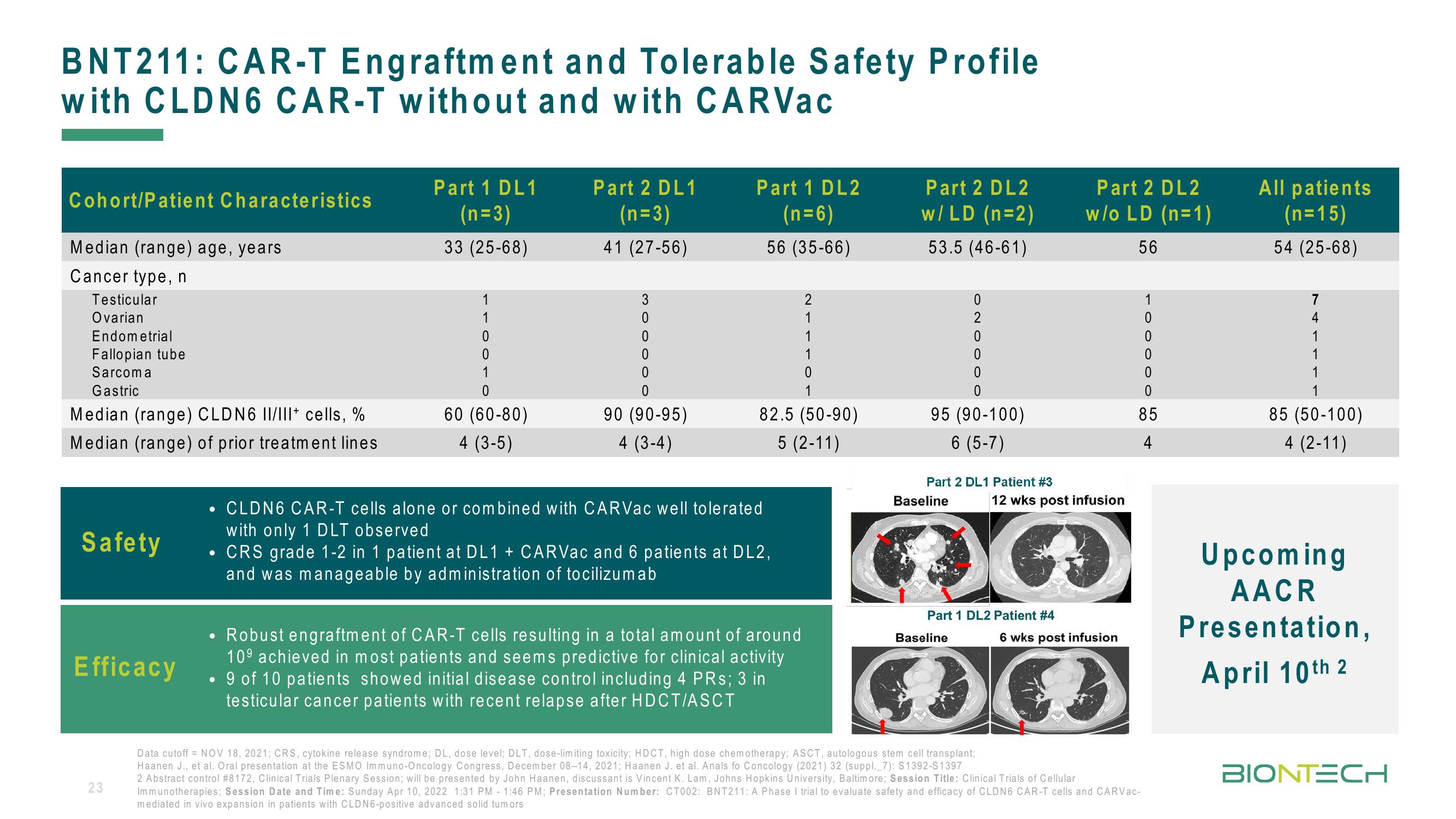
BNT211: CAR-T Engraftment and Tolerable Safety Profile
with CLDN6 CAR-T without and with CARVac
Cohort/Patient Characteristics
Median (range) age, years
Cancer type, n
Testicular
Ovarian
Endometrial
Fallopian tube
Sarcoma
Gastric
Median (range) CLDN6 II/III+ cells, %
Median (range) of prior treatment lines
Safety
Efficacy
23
●
Part 1 DL1
(n=3)
33 (25-68)
1
0
0
1
0
●
60 (60-80)
4 (3-5)
Part 2 DL1
(n=3)
41 (27-56)
3
0
0
0
0
90 (90-95)
4 (3-4)
Part 1 DL2
(n=6)
56 (35-66)
CLDN6 CAR-T cells alone or combined with CARVac well tolerated
with only 1 DLT observed
• CRS grade 1-2 in 1 patient at DL1 + CARVac and 6 patients at DL2,
and was manageable by administration of tocilizumab
Robust engraftment of CAR-T cells resulting in a total amount of around
10⁹ achieved in most patients and seems predictive for clinical activity
●
• 9 of 10 patients showed initial disease control including 4 PRs; 3 in
testicular cancer patients with recent relapse after HDCT/ASCT
2
1
1
82.5 (50-90)
5 (2-11)
1
0
1
Part 2 DL2
w/LD (n=2)
53.5 (46-61)
0
2
0
0
0
0
95 (90-100)
6 (5-7)
Part 2 DL1 Patient #3
Baseline
12 wks post infusion
ⒸO
Part 1 DL2 Patient #4
Baseline
Part 2 DL2
w/o LD (n=1)
56
6 wks post infusion
30
100OOO
85
4
Data cutoff = NOV 18, 2021; CRS, cytokine release syndrome; DL, dose level; DLT, dose-limiting toxicity; HDCT, high dose chemotherapy; ASCT, autologous stem cell transplant;
Haanen J., et al. Oral presentation at the ESMO Immuno-Oncology Congress, December 08-14, 2021; Haanen J. et al. Anals fo Concology (2021) 32 (suppl. 7): S1392-S1397
2 Abstract control #8172, Clinical Trials Plenary Session; will be presented by John Haanen, discussant is Vincent K. Lam, Johns Hopkins University, Baltimore; Session Title: Clinical Trials of Cellular
Immunotherapies; Session Date and Time: Sunday Apr 10, 2022 1:31 PM - 1:46 PM; Presentation Number: CT002: BNT211: A Phase I trial to evaluate safety and efficacy of CLDN6 CAR-T cells and CARVac-
mediated in vivo expansion in patients with CLDN6-positive advanced solid tumors
All patients
(n=15)
54 (25-68)
7
4
1
1
1
1
85 (50-100)
4 (2-11)
Upcoming
AACR
Presentation,
April 10th 2
BIONTECHView entire presentation