BioNTech Results Presentation Deck
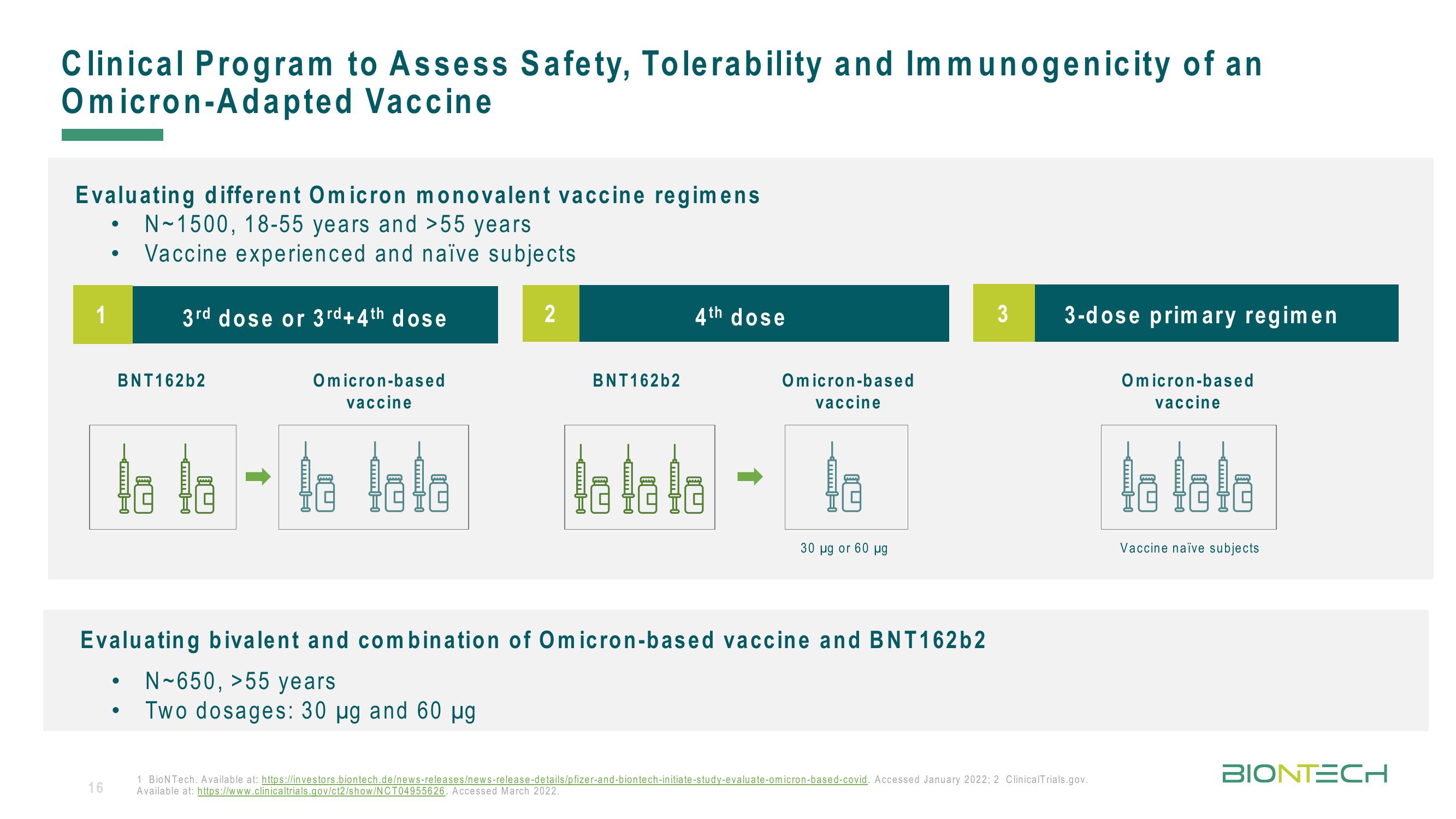
Clinical Program to Assess Safety, Tolerability and Immunogenicity of an
Omicron-Adapted
Vaccine
Evaluating different Omicron monovalent vaccine regimens
N-1500, 18-55 years and >55 years
Vaccine experienced and naïve subjects
1
●
16
Omicron-based
vaccine
to to - to toto
3rd dose or 3rd+4th dose
BNT162b2
●
●
2
BNT162b2
4th dose
to to to
Omicron-based
vaccine
Evaluating bivalent and combination of Omicron-based vaccine and BNT162b2
N-650, >55 years
Two dosages: 30 ug and 60 µg
mml=0
30 mg or 60 mg
3 3-dose primary regimen
1 BioNTech. Available at: https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-initiate-study-evaluate-omicron-based-covid. Accessed January 2022; 2 Clinical Trials.gov.
Available at: https://www.clinical trials.gov/ct2/show/NCT04955626. Accessed March 2022.
Omicron-based
vaccine
to toto
Vaccine naïve subjects
BIONTECHView entire presentation