Bausch+Lomb Results Presentation Deck
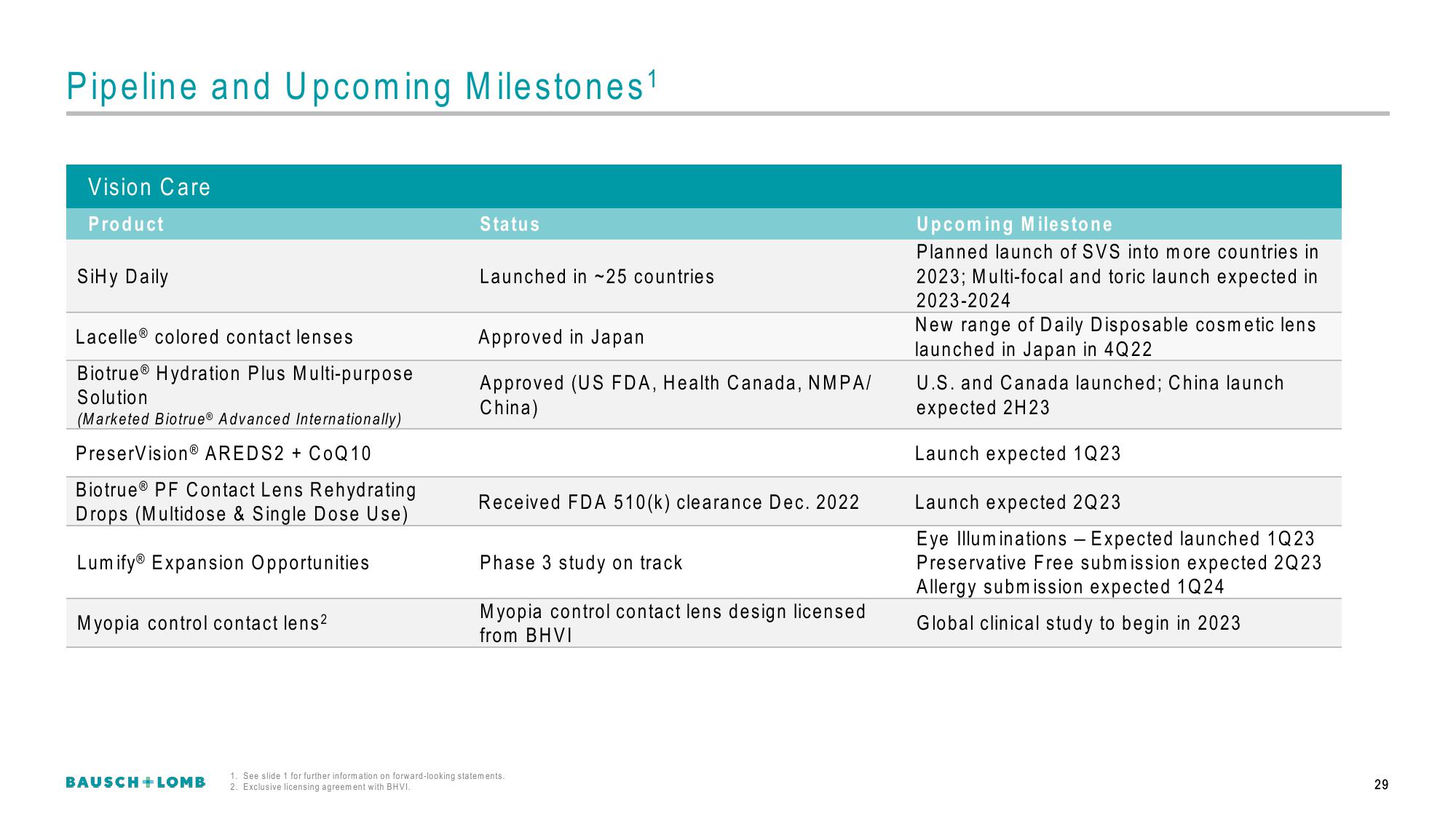
Pipeline and Upcoming Milestones¹
Vision Care
Product
SiHy Daily
Lacelle® colored contact lenses
Biotrue® Hydration Plus Multi-purpose
Solution
(Marketed Biotrue® Advanced Internationally)
PreserVision® AREDS2 + CoQ10
Biotrue® PF Contact Lens Rehydrating
Drops (Multidose & Single Dose Use)
Lumify® Expansion Opportunities
Myopia control contact lens²
BAUSCH + LOMB
Status
Launched in ~25 countries
Approved in Japan
Approved (US FDA, Health Canada, NMPA/
China)
Received FDA 510(k) clearance Dec. 2022
Phase 3 study on track
Myopia control contact lens design licensed
from BHVI
1. See slide 1 for further information on forward-looking statements.
2. Exclusive licensing agreement with BHVI.
Upcoming Milestone
Planned launch of SVS into more countries in
2023; Multi-focal and toric launch expected in
2023-2024
New range of Daily Disposable cosmetic lens
launched in Japan in 4Q22
U.S. and Canada launched; China launch
expected 2H23
Launch expected 1Q23
Launch expected 2Q23
Eye Illuminations - Expected launched 1Q23
Preservative Free submission expected 2Q23
Allergy submission expected 1Q24
Global clinical study to begin in 2023
29View entire presentation