Bausch+Lomb Results Presentation Deck
Pipeline and Upcoming Milestones¹
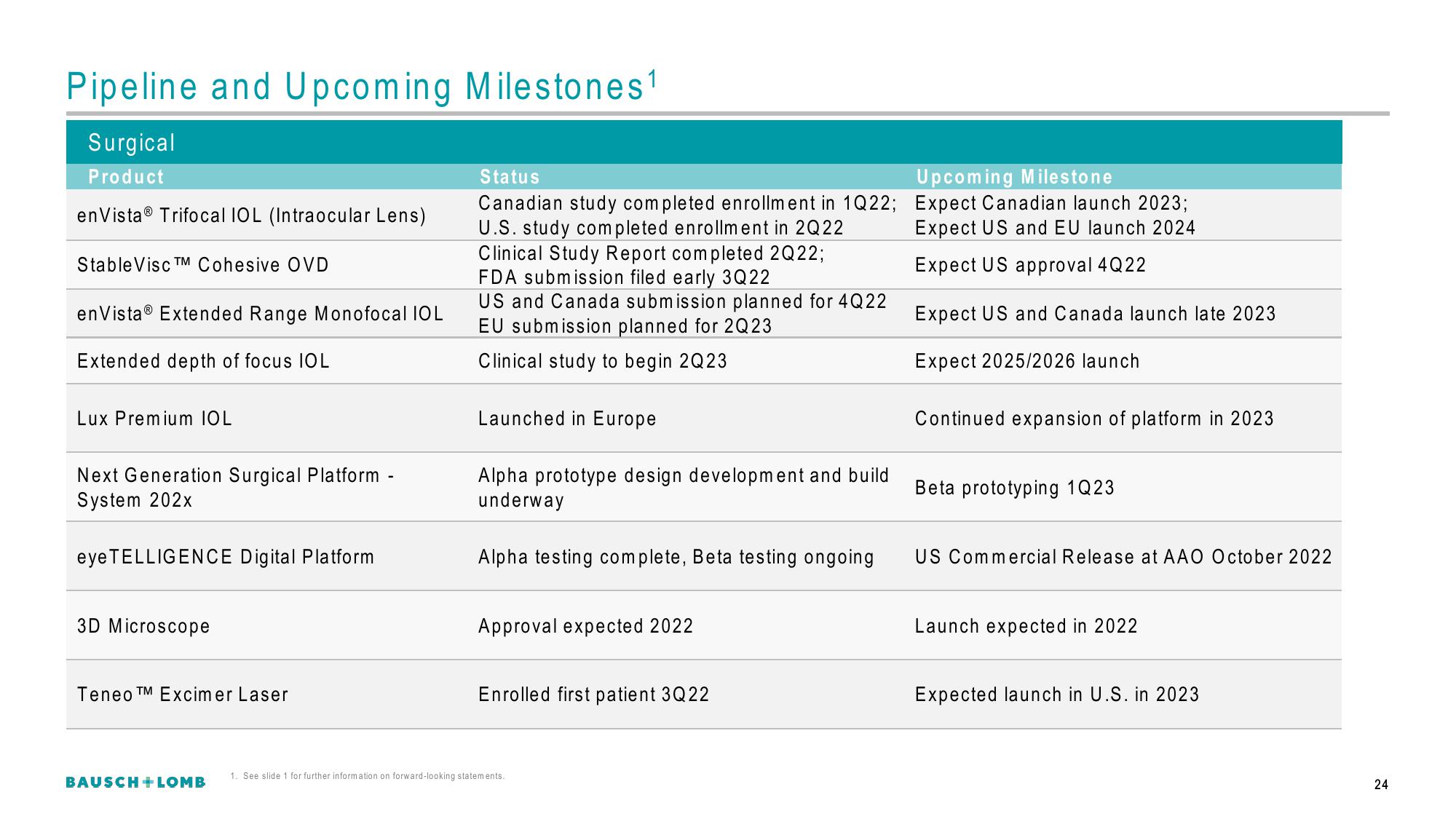
Surgical
Product
en Vista® Trifocal IOL (Intraocular Lens)
Stable Visc TM Cohesive OVD
en Vista® Extended Range Monofocal IOL
Extended depth of focus IOL
Lux Premium IOL
Next Generation Surgical Platform
System 202x
eye TELLIGENCE Digital Platform
3D Microscope
Teneo TM Excimer Laser
BAUSCH + LOMB
Status
Canadian study completed enrollment in 1Q22;
U.S. study completed enrollment in 2Q22
Clinical Study Report completed 2Q22;
FDA submission filed early 3Q22
US and Canada submission planned for 4Q22
EU submission planned for 2Q 23
Clinical study to begin 2Q23
Launched in Europe
Alpha prototype design development and build
underway
Alpha testing complete, Beta testing ongoing
Approval expected 2022
Enrolled first patient 3Q22
1. See slide 1 for further information on forward-looking statements.
Upcoming Milestone
Expect Canadian launch 2023;
Expect US and EU launch 2024
Expect US approval 4Q22
Expect US and Canada launch late 2023
Expect 2025/2026 launch
Continued expansion of platform in 2023
Beta prototyping 1Q23
US Commercial Release at AAO October 2022
Launch expected in 2022
Expected launch in U.S. in 2023
24View entire presentation